Several years after Maxwell introduced his model, which concluded light as an electromagnetic wave, the black body radiation dilemma arose. In the black body radiation we learn that a radiant energy emitted from a material body is temperature dependent and is independent of the property of the material. In the other words a material emit the same amount of energy that it absorbs. An ideal black body material absorbs all energy it gets and radiates all energy it has absorbed. The total radiation of an ideal black body is expressed as its intensity and is:
 and by Stefan-Boltzmann law is:
and by Stefan-Boltzmann law is:
Intensity spectral for the different temperature T4>T1. (Goldin, 127)
All models within the electromagnetic theory failed to be fitted into the real data in black body experiments. The best model that could get too close to the real data was Wien’s law, which is:
The results could not fit into the real data as the wavelengths were increasing.
Wien’s law vs. experiment. (Goldin, 127)
Max Planck introduced quanta in order to explain the black body radiation. He believed that each oscillator has a discrete energy states and can absorb or emit a quantum of energy  .
.
Planck’s radiation law was formed as the following equation:




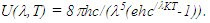




No comments:
Post a Comment