Heinrich Hertz discovered the photoelectric effect in 1887. Einstein in 1905 developed Planck’s quanta and introduced the photon.
Photoelectric-effect circuit. (Peleg, 1)
In photoelectric effect a piece of metal sheet is biased above a threshold voltage V0 and exposed to the light. A galvanometer measures the current upon light incident. When monochromatic light with high enough frequency falls on a metal electrons eject form the sheet to the anode pole, this happens instantaneously even for a very weak light intensity. This means that a change in the frequency of the radiation changes the maximum kinetic energy of electrons,  , while a change in the light intensity does not affect this energy. However the current read by the Galvanometer is intensity dependent.
, while a change in the light intensity does not affect this energy. However the current read by the Galvanometer is intensity dependent.
The following figures illustrates the properties of photoelectric effect:
Properties of photoelectric effect. (Peleg, 2)
a) If the light intensity stays constant the current proceed to its steady-state position. The transition time is about  .
.
b) The relationship between light intensity and the photoelectric current is linear.
c) The photocurrent stops at potential that reaches the maximum energy of electrons.
d) For different frequency of light there is a different maximum energy.
But in classical explanation of light the intensity of light determines the maximum energy absorbed by the electrons. However we just saw that based on Planck’s quantum theory the maximum absorbed by the electron is frequency dependent not intensity.
If E = hf is absorbed energy by the electron through light incident, then:
in the frequency form we have:
“ ” is the minimum energy to overcome atomic binding energy to generate a free electron.
” is the minimum energy to overcome atomic binding energy to generate a free electron.
“v” is the speed of the electron proportional to the frequency of the incident light.
Therefore the minimum threshold frequency is:
In Einstein’s relativity theory  where
where  is the rest mass of a particle.
is the rest mass of a particle.
If  then
then  where p is the momentum p = mv. In case of photon
where p is the momentum p = mv. In case of photon 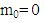 then:
then:
E = pc.
Therefore:
E = pc = hf
p =hl
These are the Planck-Einstein relations.
Based on observation it was discovered that an electron in an atomic structure could absorb and cancel discrete frequencies. This formed the Niels Bohr model of atomic structure in terms of wrapping orbits around the core of the atom. His mathematical model of orbits is:
The difference in the energy level:











No comments:
Post a Comment